quantiferon gold test bottle|quantiferon cost with lab : purchase Quantiferon-TB Gold Test Clinical Background: Quantiferon is an ELISA based interferon gamma release assay. . Exactly 1 ml in each bottle. Exactly 1 ml in each bottle. Minimum Volume Paediatric: . Quantiferon Gold samples must be submitted to the laboratory by 4pm on the day on which it is drawn. If the collection time is approaching this . Resultado da Berikut ikuti 5 langkah panduan cara daftar akun slot online dari kami, tidak sampai 3 menit kelar. 1.Akses situs dengan link alternatif yang sedang aktif. 2.Setelah berhasil login, cari tombol daftar yang disediakan. 3.Lengkapi data-data yang diminta pada form pendaftaran akun.
{plog:ftitle_list}
Resultado da O Burgo eres ti | Boneca Arteixo - Facebook . Boneca Arteixo
Collect a minimum of 6 mL of blood by venipuncture into a lithium heparin (green-top) tube. Gently mix by inverting the tube several times to dissolve the heparin. Refrigerate at 2-8° C. Note: The Centers for Disease Control and Prevention (CDC) has provided guidance on clinical considerations for tuberculosis . See more
Quantiferon®-TB Gold Plus, 4 tube collection kit • Incubated specimens • Gel barrier lithium heparin (green-top) tubes See more
What is QuantiFERON-TB Gold? QFT is a laboratory test for identifying people infected with M. tuberculosis, the bacterium that causes TB. The test identifies all stages of infection, including .Quantiferon-TB Gold Test Clinical Background: Quantiferon is an ELISA based interferon gamma release assay. . Exactly 1 ml in each bottle. Exactly 1 ml in each bottle. Minimum Volume Paediatric: . Quantiferon Gold samples must be submitted to the laboratory by 4pm on the day on which it is drawn. If the collection time is approaching this .Interpretation of QFT-Plus test results Nil (IU/ml) TB1 minus Nil (IU/ml) TB2 minus Nil (IU/ml) Mitogen minus Nil (IU/ml) QFT -Plus Result Re sult interpretation ≤8.0 ≥0.35 and ≥25% of Nil Any Any Positive M. tuberculosis infection likely Any ≥0.35 and ≥ 25% of Nil <0.35 or ≥0.35 and <25% of Nil <0.35 or ≥0.35 The TB blood test (interferon gamma release assay [IGRA]) mixes a patient’s blood samples with peptides that simulate antigens derived from TB bacteria and with controls. . QuantiFERON®-TB Gold Plus (QFT-Plus) T .
what is quantiferon gold test
Determining an approach Targeted testing. CDC and the U.S. Preventive Services Task Force (USPSTF) recommend testing people who are at increased risk for TB infection. Testing for TB infection is a routine and integral part of health care for patients with increased risk for TB.. People who are at low risk generally should not be tested because the predictive value .
The QuantiFERON TB test has been shown to be accurate in HIV-positive individuals with moderately advanced disease, . Guidelines for using the QuantiFERONR-TB Gold Test for detecting Mycobacterium tuberculosis Infection, United States. 2005;54(NoRR-15). REMICADE® [package insert]. Horsham, PA: Janssen Biotech, Inc. 2013.Guidelines for Using the QuantiFERON ®-TB Gold Test for Detecting Mycobacterium tuberculosis Infection, United States. Please note: An erratum has been published for this article. To view the erratum, please click here.. Prepared by Gerald H. Mazurek, MD, John Jereb, MD, Phillip LoBue, MD, Michael F. Iademarco, MD, Beverly Metchock, PhD, Andrew Vernon, MD
7 QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert 01/2023 In MTB infection, CD4+ T cells play a critical role in immunological control through their secretion of the cytokine IFN-γ. Evidence now supports a role for CD8+ T cells participating in the host defense to MTB by producing IFN-γ and other soluble factors, which activate4 QuantiFERON-TB Gold Plus FAQ for Health Professionals 07/2017 Questions and answers QuantiFERON-TB Gold Plus (QFT-Plus®) is a whole-blood test that measures the cell-mediated immune response of tuberculosis (TB) infected individuals. Approved by the US Food and Drug
turnaround time for quantiferon gold
The test is suitable for use with all patients at risk of latent tuberculosis infection or suspected of having TB disease, regardless of age, sex, ethnicity, therapy or immune status. . TB quantiferon Gold test; Mycobacterium and TB investigations; Sample requirements. Blood in TWO 6 mL lithium heparin tube.
These Frequently Asked Questions (FAQs) relate to the QuantiFERON-TB Gold Plus (QFT®-Plus) assay. The answers provided are meant to act as a guide only. Refer to your QuantiFERON-TB Gold Plus Package Insert for approved test procedures, as well as for all other enquiries relating to the use or performance of the assay.Main Laboratory . The Doctors Laboratory The Halo Building, 1 Mabledon Place London, WC1H 9AX, UK . Tel: +44 (0)20 7307 7373 Email: [email protected]/TB Culture. Microbiology. AAFB Microscopy -24hrs. Rapid Liquid Culture -35days. Solid Culture- 8 weeksThe QuantiFERON ®-TB Gold test (QFT-G) is a whole-blood test for use as an aid in diagnosing Mycobacterium tuberculosis infection, including latent tuberculosis infection (LTBI) and tuberculosis (TB) disease. This test was approved by the .
quantiferon lab test near me
Gold top tube. Contains clot activator and serum gel separator – used for most chemistry and immunology laboratory tests. Mix 8 to 10 times immediately after collection. Let stand for 20 minutes before centrifuging. Contains lithium heparin and a plasma gel separator – used for . Mix 8 to 10 times immediately after collection to preventQuantiFERON-TB Gold Collection Processing Instructions 1. Collect 1 mL of blood into each of the 3 tubes - Standard Altitude: QuantiFERON-QTB Gold In-Tube Collection Kit (T628) Tubes fill slowly. Use of a syringe may ensure correct blood volume. When the tube is upright, blood must meet the small black mark on the label.
The Quantiferon-TB Gold test (QFT-G) is a whole-blood test for use as an aid in diagnosing Mycobacterium tuberculosis infection, including . nil, tb antigen, and mitogen. The device, as modified, will be marketed under the trade name quantiferon-tb gold in-tube and is indicated for use as an in vitro diagnostic test using a peptide cocktail .Test QuantiFERON TBC - Procedura de recoltare, valori de referinta si interpretare rezultate | MedLife Mergi la conţinutul principal Call Center . QuantiFERON, 3 Dec. 2021, www.quantiferon.com “Quantiferon TB Gold Test - About, Preparation, Test Results & More.” Portea.com, 2019, www.portea.com “QuantiFERON-TB Gold - QuantiFERON .
QuantiFERON-TB Gold Plus Test. Bucuresti, Pitești, Ploiești, Vâlcea, Buzău, Târgoviște, Urziceni, Alexandria și Giurgi: recoltarea se efectuează luni – joi: 07:00 – 14:00. Cluj: recoltarea se efectuează în oricare locație Synevo din Cluj luni – vineri: 07:00 – 13:00. Galați: recoltarea se efectuează în Laborator și centru de recoltare Galați (B-dul George Cosbuc nr. 251 .
Written by Associate Professor Owen Harris. Interferon Gamma Release Assays (IGRA)s are in vitro blood tests of the cell-mediated immune system.The QuantiFERON-TB Gold (QFT) assay is an IGRA measuring T-cell release of IFN- γ following stimulation by antigens specific to Mycobacterium tuberculosis complex.. The test. The QFT assay consists of four tubes: the .Posted: Feb 22nd, 2022 at 12:00AM - by Ashlee Arnold/Vice President The QuantiFERON TB Gold Test is a medical test that screens for tuberculosis (TB). It’s an Interferon Gamma Release Assay (IGRA) test that involves taking a blood sample which will then be analyzed in a lab. The QuantiFERON TB Gold test was approved by the Food and Drug Administration in 2011 and . Updated versions of 'QuantiFERON®: blood collection, storage and transportation guidelines' and 'QuantiFERON®: TB Gold plus blood collection tubes order form'. 17 October 2016 Updated form and .A negative QuantiFERON-TB Gold Plus (QFT-Plus) result does not preclude the possibility of Mycobacterium tuberculosis infection or tuberculosis disease. False-negative results can be due to the stage of infection (eg, specimen obtained prior to the development of cellular immune response), comorbid conditions that affect immune functions, incorrect handling of the blood .
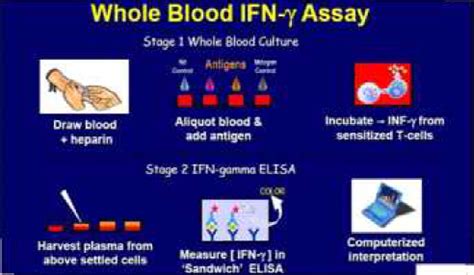
QuantiFERON -TB Gold In-Tube (QFT) este un test pentru aprecierea răspunsului imun mediat celular faţă de antigenele unor peptide care simulează proteinele micobacteriene (ESAT-6, CFP-10). Persoanele infectate cu Mycobacterium tuberculosis prezintă de obicei, limfocite care recunosc aceste antigene.4 QuantiFERON-TB Gold (QFT) ELISA Package Insert 08/2016 1. Intended Use QuantiFERON-TB Gold (QFT®) is an in vitro diagnostic test using a peptide cocktail simulating ESAT-6, CFP-10, and TB7.7(p4) proteins to stimulate cells in heparinized whole blood. Detection of interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) isNo. BCG does not affect the results of the QuantiFERON®-TB Gold test. While it’s true that BCG can give you a false positive skin test, the proteins used in the QuantiFERON®-TB Gold test are not found in BCG, so the BCG will not give you a false positive QuantiFERON®-TB Gold test. What kind of results do you get with the QuantiFERON®-TB .LIAISON QuantiFERON-TB Gold Plus Test receives FDA approval. Find out more QIAGEN's QuantiFERON-TB passes milestone 60 million tests sold in global fight against tuberculosis, annual run rate exceeds 12 million tests. Find out more Previous Next. Information for patients .
quantiferon gold testing near me
quantiferon gold test cost
quantiferon gold quest test code
Você tem 5 maneiras de ir de Lomas de Solymar para Beto .
quantiferon gold test bottle|quantiferon cost with lab